- 01: Introduction
- 02: History
- 03: Propellants, Firearms, and Ammunition Development
- 04: Modern Firearms Manufacture
- 05: Small Arms Ammunition
- 06: Evidence Handling Procedures
- 07: Equipment and Instrumentation
- 08: Examination of Firearms
- 09: Cartridge and Shotshell Examination
- 10: Characterization and Evaluation of Fired Projectiles
- 11: Bullet Comparison and Identification
- 12: Gunshot Residue and Distance Determination
- Introduction
- Objectives
- AFTE Knowledge and Ability Factors
- Powders and Residues
- Examination
- Distance Determination
- Significance of Results
- Contact Shot
- Nitrite Residues
- Vaporous Lead Residues
- Residues Consistent with the Passage of a Bullet
- Residues Consistent with the Discharge of a Firearm
- Interpretation Challenges
- Reproduction of Results
- Nitrite Residue Patterns
- Shot Patterning
- Chemical Testing
- Shot Pattern Reproduction
- Selected Bibliography
- 13: Toolmark Identification
- 14: Communicating Results
- Resources


Chemistry
Home > Gunshot Residue and Distance Determination > Examination > Chemical Testing > Chemistry
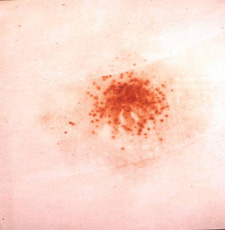
Modified Griess Test results at 3 inches
In the Modified Griess Test, a series of chemical reactions result in the conversion of any nitrite compounds that may be present on an item, such as victim clothing, into a bright orange dye in a chromophoric reaction. These dye pigments are preserved in an emulsion-coated test medium (desensitized photographic paper or printer paper) for later side-by-side comparison with known distance firings of similar residue patterns.
The chemistry of the Modified Griess Test is comprised of the following process:
- Nitrite residues are exposed to an acetic acid solution and heat to form nitrous acid.
- The nitrous acid combines with sulfanilic acid in the test medium to form a diazonium compound of sulfanilic acid.
- The diazonium compound couples with the alpha-naphthol (also in the test medium) to form a bright orange water-soluble azo (nitrogen-bearing) dye.




